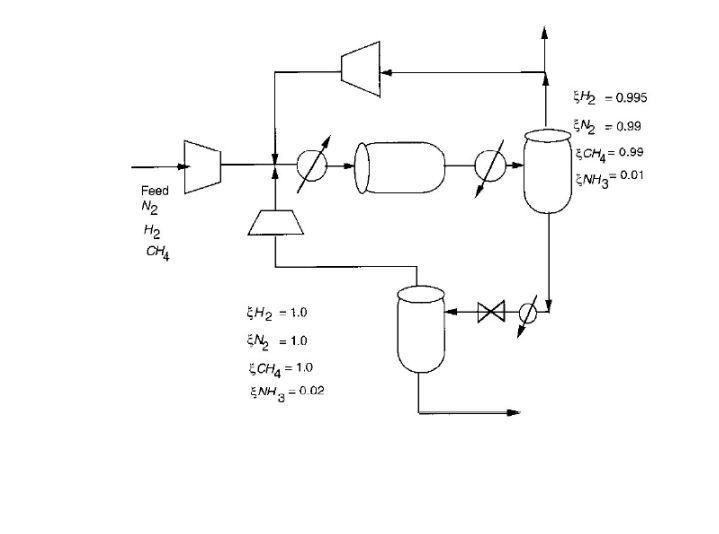
Chemical Engineering Question on Mass Balance:
Consider the ammonia process given below. A feed of 20% N2, 78% H2 and 2% CH4 is mixed with two recycle streams and enters a reactor. Here, conversion per pass of N2 to NH3 is 45% according to the reaction:
N2 + 3H2 –> 2NH3
The ammonia product is recovered by flashing the reactor effluent in two stages and recycling the overhead vapour. If the purge fraction is 5% for the high pressure recycle, what is the methane concentration in the reactor feed?