Question
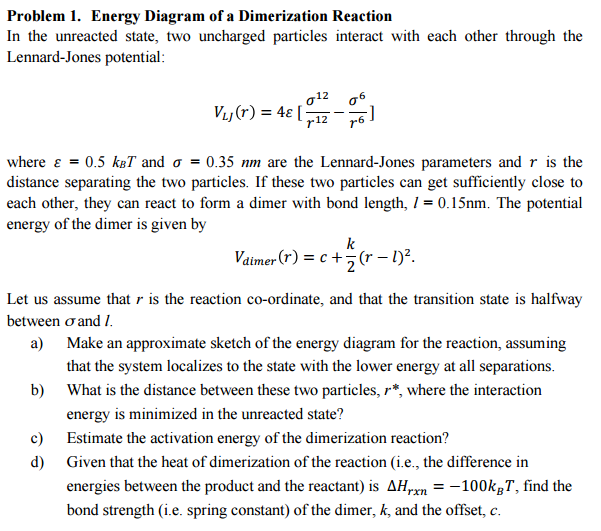
Show transcribed image text Energy Diagram of a Dimerization Reaction In the unreacted state, two uncharged particles interact with each other through the Lennard-Jones potential: VLJ (gamma) = 4 epsilon [sigma^12/gamma^12 – sigma^6/gamma^6] where epsilon = 0.5 KBT and sigma = 0.35 nm are the Lennard-Jones parameters and gamma is the distance separating the two particles. If these two particles can get sufficiently close to each other, they can react to form a dimer with bond length, l = 0.15nm. The potential energy of the dimer is given by V dimer (gamma) = C + K/2(gamma – l)^2 Let us assume that r is the reaction co-ordinate, and that the transition state is halfway between sigma and l. Make an approximate sketch of the energy diagram for the reaction, assuming that the system localizes to the state with the lower energy at all separations. What is the distance between these two particles, gamma*, where the interaction energy is minimized in the unreacted state? Estimate the activation energy of the dimerization reaction? Given that the heat of dimerization of the reaction (i.e., the difference in energies between the product and the reactant) is delta H gamma x n = -100kBT, find the bond strength (i.e. spring constant) of the dimer, K, and the offset, c.




